Extensive molecular analysis of large B-cell lymphomas (LBCL) have provided basis for new concepts of lymphoma taxonomy. These include stages of differentiation, phenotype (cell of origin) as well as genotype (including, but not confined to gene rearrangements) and immune microenvironment. The importance of the tumor microenvironment has recently regained focus due to development of immune checkpoint-inhibitors such as PD1/PD-L1 inhibitors, and LBCL may according to the new subtypes be stratified as microenvironment "inflamed" or "non-inflamed", which may hold prognostic as well as predictive value.
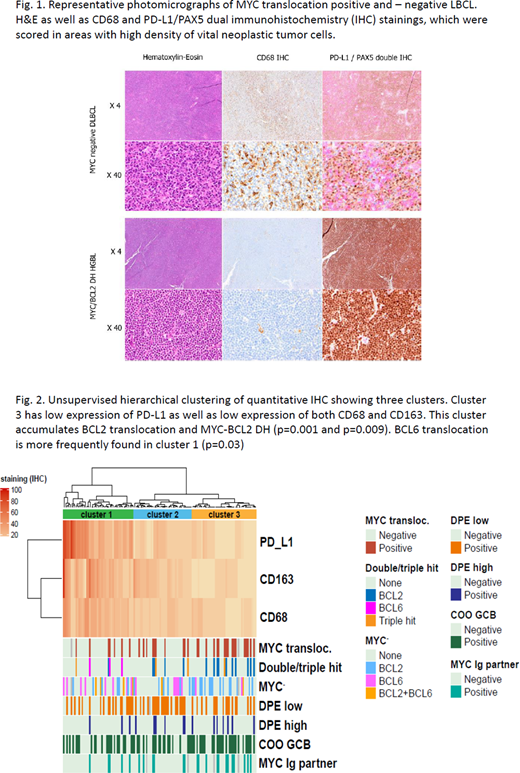
We investigated the composition of the tumor microenvironment in LBCL with or without MYC-translocation regarding PD-L1 expression and the infiltration of tumor-associated macrophages (TAMs). 126 patient samples were studied in a cohort enriched for MYC translocated tumors with 34 samples carrying this translocation. Demonstration of intratumoral distribution and cellular source of PD-L1 was enabled by immunohistochemical (IHC) dual staining specifically highlighting PD-L1 expression in lymphoma B-cells with antibodies against PD-L1 and Pax-5. Additional IHC with antibodies against CD68 and CD163 identified tumor associated macrophages (TAMs). All IHC stainings were quantitated independently and blinded by two consultant hematopathologists in 10% intervals of all tumor cells both neoplastic B-cells, immune cells, and stromal cells. We found that CD68 positive TAMs were the main source of PD-L1 protein expression in contrast to lymphoma B-cells which rarely expressed PD-L1 (Fig.1). Quantitative IHC demonstrated a significant correlation between CD68 and PD-L1 protein expression. CD68 positive TAMS and thereby also PD-L1 expression was significantly lower in MYC translocated lymphomas compared to non-translocated. The correlation was significant for both MYC single-hit (SH) and MYC double-hit (DH) with concurrent BCL2 and/or BCL6 translocation, the latter now termed High-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements (HGBL-DH/TH).
For a more detailed analysis we next performed unsupervised cluster analysis of PD-L1, CD68 and CD163 quantitative IHC data, which demonstrated three significant clusters (Clusters 1-3) defined by expression of the three biomarkers (Fig. 2). Cluster 3 consisted of patient samples with significantly lower expression of PD-L1, CD68 and CD163, but also significantly higher prevalence of BCL2-translocation and MYC/BCL2-DH compared to the other two clusters. In cluster 1 we found a significant accumulation of BCL6 translocated tumors. This cluster in contrast had the highest protein expression of PD-L1, CD68 and CD163.
Our data which were based on morphological analysis, immunophenotyping and genotyping by FISH, were in line with new concepts of LBCL taxonomy integrating genetic, phenotypical and immunological characteristics with identification of new subgroups where MYC-translocation and MYC/BCL2 DH may identify a noninflamed subtype. These findings may furthermore hold significant predictive value especially regarding immune checkpoint blockade therapy, but further molecular characterization should be done to substantiate this hypothesis.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal